Revision of the fossil figitid wasps (Insecta: Hymenoptera: Cynipoidea) described from compression deposits during the first half of the 20th century
Revisión de las avispas figítidas fósiles (Insecta: Hymenoptera: Cynipoidea) descritas de depósitos de compresión durante la primera mitad del siglo XX
J. Pujade-Villar1, E. Peñalver2
1Universitat de Barcelona, Facultat de Biologia, Departament de Biologia Evolutiva, Ecologia i Ciències Ambientals, Avda. Diagonal, 645, E-08028 Barcelona. Spain. Email: jpujade@ub.edu. ORCID ID: https://orcid.org/0000-0001-7798-2717
2Instituto Geológico y Minero de España (Museo Geominero), C/ Cirilo Amorós, 42, entreplanta, E-46004 Valencia. Spain. EMail: e.penalver@igme.es (corresponding author). ORCID ID: https://orcid.org/0000-0001-8312-6087
| |
ABSTRACT
The holotypes of the Cenozoic fossil wasps attributed to the family Figitidae, which were described in the first half of the 20th century by Charles T. Brues and Georg Statz from Florissant (USA) and Rott-am-Siebengebirge (Germany) sites respectively, have been restudied. The following new taxonomic changes are proposed: Palaeogronotoma? sola (Brues) n. comb., Aulacidea rotundata (Statz) n. comb., A. plana (Statz) n. comb. and A. spiniger (Statz) n. comb. The three last species listed have changed their taxonomic position, as the extant genus Aulacidea belongs to family Cynipidae, not Figitidae as was considered originally in 1938. The revision of one Baltic amber fossil species originally described in 1919, shows that it has a unique set of characters within Cynipidae, allowing the description of a new tribe: Kinseycynipsini n. tribe. Taxonomical comments about other three fossil cynipid species are provided. The correct taxonomical placement of the figitid and cynipid fossil species described ca. one century ago is important for understanding the evolution of these two cynipoid families, which play an important ecological role in the extant terrestrial ecosystems. In addition, updated lists of figitid and cynipid fossil species known are also provided.
Keywords: Cenozoic; Germany; USA; compression fossils; Hymenoptera; Figitidae; Cynipidae; taxonomical revision.
|
| |
RESUMEN
En la presente investigación se revisan los holotipos de las avispas fósiles del Cenozoico atribuidas a la familia Figitidae, las cuales fueron descritas en la primera mitad del siglo XX por Charles T. Brues y Georg Statz provenientes de los yacimientos de Florissant (EE.UU.) y Rott-am-Siebengebirge (Alemania), respectivamente. Se proponen los siguientes cambios taxonómicos: Palaeogronotoma? sola (Brues) n. comb., Aulacidea rotundata (Statz) n. comb., A. plana (Statz) n. comb. y A. spiniger (Statz) n. comb. Los cambios taxonómicos para las tres últimas especies indicadas han implicado su emplazamiento en el género actual Aulacidea, de la familia Cynipidae, y por lo tanto no pertenecen a la familia Figitidae como se consideró originalmente en 1938. La revisión de una especie fósil originalmente descrita en 1919, conservada en ámbar báltico, muestra que posee un conjunto único de caracteres para la familia Cynipidae, permitiendo la descripción de una nueva tribu: Kinseycynipsini n. tribe. Se hacen indicaciones taxonómicas referidas a otras tres especies de cinípidos fósiles. La adscripción taxonómica correcta de las especies fósiles de figítidos y cinípidos descritas hace alrededor de un siglo es importante para entender la evolución de estas dos familias de cinipoideos, las cuales desempeñan un importante papel ecológico en los ecosistemas terrestres actuales. También se proporcionan listas actualizadas de las especies fósiles de figítidos y cinípidos conocidas.
Palabras clave: Cenozoico; Alemania; Estados Unidos de América; fósiles de compresión; Hymenoptera; Figitidae; Cynipidae; revisión taxonómica.
|
IntroductionTOP
The superfamily Cynipoidea, as a whole, likely appeared in the Jurassic, however the fossil evidence for its earliest evolution has yet not been found (Grimaldi & Engel, 2005). Superfamily Cynipoidea comprises eight families, three of which have been recently described or re-described by Liu et al. (2007) as fossil taxa lacking extant representatives.
Ronquist (1995) established the “microcynipoids” that comprise the extinct family Gerocynipidae and the living families Figitidae and Cynipidae, according to the revision accomplished by Liu et al. (2007). Representatives of Gerocynipidae are the oldest microcynipoids. They were found in Upper Cretaceous (Santonian / mid-Campanian; see Herman, 2011) mudstones from the Obeshchayushchiy Creek in the Russian Far East, and according to Kovalev (1994, 1995) they are considered to be the predecessors of gall-makers. This group is morphologically characterized by having very short propodeum, an extended ovipositor (with coiling of 2nd valvifer at 360º) and a rectangular metapleuron (Kovalev, 1994, 1995). Ronquist (1995, 1999) mentioned that an external character to differentiate both families is the direction of Rs+M vein in forewings; in Cynipidae it is usually directed to the middle of basal vein but to the end of basal vein in Figitidae. This character is problematic because the vein Rs+M is not always present and in some Cynipidae it is close to the lower part of the basal vein (Pujade-Villar, 2002).Figitidae comprise 13 subfamilies (Liu et al., 2007; Ros-Farré & Pujade-Villar, 2007; Buffington & Liljeblad, 2008). Two of them are only known from the fossil record, as the extinct family Rasnicynipidae was transferred to Figitidae and reclassified as the basal subfamily Rasnicynipinae (Grimaldi & Engel, 2005; Liu et al., 2007). On the other hand, the family Cynipidae comprises 2 subfamilies (Ronquist, 1995, 1999), one of them is currently extinct and was morphologically similar to the Figitidae, making the taxonomical distinction somehow difficult.
Cynipidae are gall-inducers or inquilines of galls. In contrast, Figitidae are larval parasitoids or hyperparasitoids of diverse orders of insects: Diptera, Hemiptera (Homoptera), Neuroptera and Hymenoptera (Fergusson, 1986; Gauld & Bolton, 1988; Goulet & Huber, 1993; Fergusson, 1995; Liu et al., 2007).
Cynipidae have been recently re-organized (Ronquist et al., 2015). Prior to that study the number of accepted tribes was 8; currently 12 extant tribes are accepted (Aylacini, Aulacideini, Phanacidini, Diastrophini, Diplolepidini, Pediaspidini, Eschatocerini, Cynipini, Qwaqwaiini, Synergini, Ceroptresini and Paraulacini).
The fossil record of the families Figitidae and Cynipidae is very scarce (Liu et al., 2007). The first fossil records are from the upper Cretaceous, Figitidae from the Turonian (93.9–89.8 ± 0.3 MY; MY = million years), younger (Upper Cretaceous: Campanian, 83.6 ± 0.2–72.1 ± 0.2 MY) for Cynipidae. On the other hand, Archaeocynipidae described by Rasnitsyn and Kovalev (1988), is not considered here since it was eliminated from the Cynipoidea by Ronquist (1999) by suggesting that it was more closely related to Diapriidae (Proctotrupoidea). Some of the most modern descriptions (e.g. Liu et al., 2007) belong to specimens preserved in amber, allowing detailed descriptions of them; on the other hand, specimens in compression deposits lack some minute morphological characters, and most of them were described in the beginning of the palaeoentomology. The last figitid taxon described as a compression fossil is an eucoiline from the Rubielos de Mora outcrop, a Miocene lacustrine deposit in Spain (Peñalver et al., 2013). All fossil compressions of Cynipidae were described during the early 20th century.
During the first half of the 20th century four fossil species from two compression deposits were described, one from the Late Eocene of Florissant (USA) and three from the Oligocene–Miocene boundary of Rott-am-Siebengebirge (Germany). These species were originally assigned to the genus Figites (Figites solus Brues, 1910; Figites? planus Statz, 1938; Figites? rotundatus Statz, 1938 and Figites? spiniger Statz, 1938, respectively). The species described by Statz (1938), were considered by Kovalev (1994, 1995) and Liu et al. (2007), without examination of the types, as belonging to the genus Hodiernocynips Kovalev, 1994 (Cynipidae: Hodiernocynipinae). The genus Hodiernocynips was established without figuration and Kovalev assigned the three species of Statz to this genus, but this author was not completely sure of such taxonomical placement. Descriptions by Brues and Statz were not detailed enough and since then the cynipoid taxonomy has been in constant revision, with very different arrangements of genera and families. Here we revise the holotypes of these four Cenozoic species in order to re-describe them, and to determine their taxonomical position in the current cynipoid taxonomy. Also we comment the taxonomical position of other fossil species.
Material and MethodsTOP
The specimens restudied are housed in the American Museum of Natural History in New York (holotype A60 of Figites solus) and the Natural History Museum of Los Angeles in California (holotypes nº 3953, nº 3954 and nº 3955 of Figites? planus, F.? rotundatus and F.? spiniger, respectively; all of them are constituted by part and counterpart). The Natural History Museum of Los Angeles acquired the complete collection of Georg Statz, who published several large papers on Rott palaeofauna between 1936 and 1950.
The holotype specimen of Figites solus was discovered in shales from Florissant in USA, and the other three holotype specimens of Figites? planus, F.? rotundatus and F.? spiniger, come from Rott-am-Siebengebirge in Germany.
The Florissant palaeolake constitutes the first major fossil insect deposit (Florissant Fm.) to be studied in North America; it is located in the Colorado State and has been dated as Late Eocene (see Evanoff, 2001 and Antropov et al., 2014). Their fine-grained shales preserve insects and other organisms in exquisite detail as carbonaceous films; it is a typical Konservat-Lagerstätte. Nearly 200 families and 1,100 species of insects (including abundant hymenopteran species), and 140 species of plants, are known from this deposit (Grimaldi & Engel, 2005), which is the type locality for several of them. Some of the plants have relatives found only in Southeast Asia today. Apart of the American Museum of Natural History, the Museum of Comparative Zoology (Harvard University) and the University of Colorado housed collections of fossils from this deposit. The most recent reviews of the Florissant palaeobiota are found in Meyer (2003) and Grimaldi & Engel (2005).
The lacustrine deposit of Rott-am-Siebengebirge, near Bonn, is one of the most studied palaeoentomological outcrops in Europe. As in the case of Florissant, the fine preservation of its fossils in fine-grained shales includes it between the fossil sites named Konservat-Lagerstätten. Age of the deposit is not well constrained, being either latest Oligocene (Chattian) or earliest Miocene (Aquitanian) (Grimaldi & Engel, 2005). Most likely, the fossil wasps come from the assemblage zone 1a. Conventional K-Ar dating of sanidines from Siebengebirge trachytes (zone 1b, Offenkaule exposure), that overlies directly zone 1a, yielded ages between 25.2 ± 0.6 and 26.4 ± 0.8 MY (Todt & Lippolt, 1980; Mertz et al., 2007: p. 357).
The four specimens show overall deterioration including crazing. There are not original photographs, but the original drawings clearly were made from well-contrasted specimens that deteriorated after they were found and studied. Most likely, the carbonaceous films that constitute these typical compression fossils slowly cracked and/or cleared due to bacterial decomposition, oxidation, exposure to light, and fluctuations in heat and humidity. Similar changes have been detected in several dipteran specimens from the Spanish Ribesalbes outcrop (Miocene), when compared to ancient photographs, present in two old collections (ca. 100 years old) at the Museo Nacional de Ciencias Naturales and Museo Geominero, both in Madrid (see Peñalver, 2002 and Peñalver et al., 2016). In consequence, Brues and Statz had more morphological details available for their studies, which is especially evident in the case of the antennal segmentation, currently very difficult to observe in the specimens. In some cases, the specimens belonging to Rott were coated with thin layers of a hardener product which preserved them for long-term study. These thin layers did not prevent the current study of the specimens.
The holotype A60 of Figites solus was reviewed from detailed photographs kindly taken by Mrs. Bushra Hussaini (American Museum of Natural History) for us, and with the assistance of Dr. David Grimaldi, from the same institution, to determine some tiny morphological characters. The holotype specimens from Rott-am-Siebengebirge were shipped by the Natural History Museum of Los Angeles to us for a direct restudy. All these specimens were examined, drawn and photographed dried and covered with alcohol, permitting the translucency of the layer of sediment that cover them. Also, the alcohol enhances the contrast of the carbonaceous film, which constitutes the specimen, with respect to the matrix. Drawings were made with a camera lucida, integrating in the same drawing the characters present in both the part and counterpart of each specimen. Photomicrography of the Rott specimens used a digital camera attached to a stereomicroscope Olympus BX51.
We follow the current terminology for morphological structures (Liljeblad & Ronquist, 1998; Melika, 2006). Abbreviations for forewing venation follow Ronquist & Nordlander (1989), and cuticle sculpture terminology follows that of Harris (1979). Width of the forewing radial cell was measured from the margin of the wing to the Rs vein. Abbreviations used here include: F1–F12 for the 1st and subsequent flagellomeres.
Systematic palaeontologyTOP
Class Insecta Linnaeus, 1758
Order Hymenoptera Linnaeus, 1758
Superfamily Cynipoidea Latreille, 1802
Family Figitidae Thomson, 1862
Subfamily Eucoilinae Thomson, 1862
Tribe Diglyphosemini Belizin, 1961
Genus Palaeogronotoma Peñalver, Fontal-Cazalla & Pujade-Villar, 2013
Palaeogronotoma? sola (Brues) n. comb.
Figites solus Brues, 1910
(Fig. 1)
|
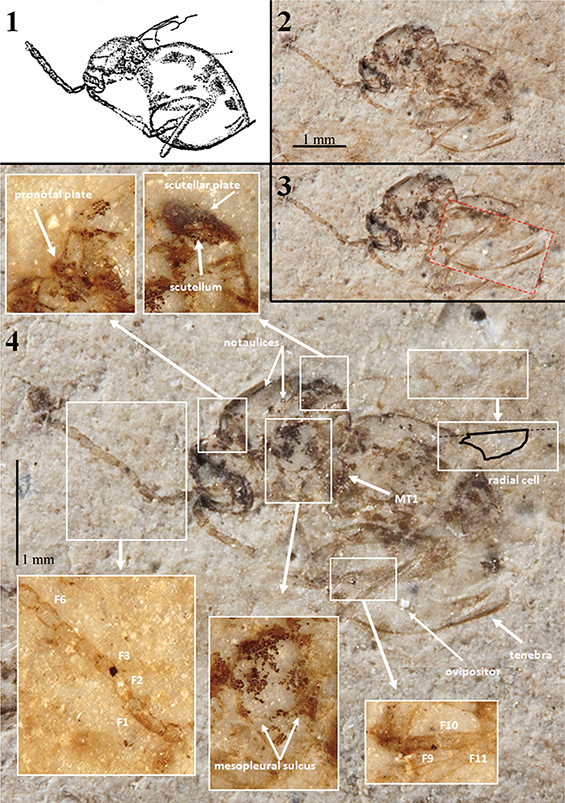 Fig. 1.—Holotype specimen (A60) of Palaeogronotoma? sola n. comb., from Florissant, USA. 1) Original drawing made by Brues (1910). 2) Photograph of the holotype under dry conditions. 3) Same photograph pointing the ventral part of metasoma in the correct position. 4) Similar photograph; insets contain photographs under alcohol showing important morphological characters. Photographs 2–4 taken by Mrs. Bushra Hussaini (Courtesy of AMNH). Abbreviations: F = Flagellomere, MT1 = first tergum of the metasoma. Fig. 1.—Holotype specimen (A60) of Palaeogronotoma? sola n. comb., from Florissant, USA. 1) Original drawing made by Brues (1910). 2) Photograph of the holotype under dry conditions. 3) Same photograph pointing the ventral part of metasoma in the correct position. 4) Similar photograph; insets contain photographs under alcohol showing important morphological characters. Photographs 2–4 taken by Mrs. Bushra Hussaini (Courtesy of AMNH). Abbreviations: F = Flagellomere, MT1 = first tergum of the metasoma.
|
|
Occurrence: Florissant (Colorado, USA) fossil beds, Florissant Formation. Late Eocene (see Evanoff, 2001 and Antropov et al., 2014). See Meyer (2003) and Grimaldi & Engel (2005).
Holotype: Alate female adult, holotype A60, in a slab of paper shale, housed in the American Museum of Natural History (New York, USA). Reviewed by the authors after the detailed photographs sent by David Grimaldi and Bushra Hussaini (AMNH), and with the assistance of David Peris (UB).
Redescription: Female. Length 2.7 mm. Antennae 13-segmented, slender, club very slightly thickened formed by 6 segments; pedicel shorter and narrower than scapus; F1 long, fully twice as long as F2 which is equal to the pedicel; F2=F3=F4; F6 and following forming the club about equal, ovate in form (David Peris, pers. com.). Mesosoma seen in latero-dorsal view with anterior (basal) part of pronotal plate distinctly protruding anteriorly; notaulices percurrent; scutellar plate differentiate; mesopleura with a complete and straight transversal carina or metapleural sulcus. Metasoma sub-sessile, about as long as the head + mesosoma together, apparently not pubescent at the base, although this character may have been lost in the process of preservation. Legs rather stout for this group. Forewings hyaline, radial cell closed (David Grimaldi, pers. com.) about two and half times as long as wide; Rs+M not visible (David Grimaldi, pers. com.). Ovipositor partially (including tenebra) visible.
Comments: Brues (1910) described this specimen as Figites solus (family Cynipidae) and considered it as a male. After examining the holotype, the metasoma corresponds to a female (Figs. 1.1–1.3). The genitalia is emerged, possibly due to body swelling by wetting/decomposition during the early taphonomic processes (Fig. 1.2) (Peñalver, 2002). Brues (1910) indicated “the probable absence of a cupuliform shape to the scutellum”, nevertheless this feature is clearly visible in the laterally preserved specimen, and after our examination we place it in the Eucoilinae. Forshage & Nordlander (2008) classified the specimen within the tribe Diglyphosemini, as is the only tribe that has notaulices impressed as deep furrows (Fig. 1.4), percurrents or not. However, Figites solus differs from the species included in this tribe in its anterior (basal) part of the pronotal plate which distinctly protrudes anteriorly (Fig. 1.4). The morphological characters approach this species to Palaeogronotoma, a recently described fossil genus from Rubielos de Mora site in Spain (Peñalver et al., 2013). Differs on size (P.? sola n. comb. is longer), and length and shape of radial cell. Furthermore, Florissant site (Late Eocene) is older than Rubielos de Mora site (Lower Miocene). The bad preservation of the holotype prevents seeing some important characters as are the Rs+M vein and the sculpturations of the metasoma and mesopleura. For that reason its placement in the genus Palaeogronotoma is tentative.
Note on palaeobiology: The palaeobiology of this species is unknown, but all extant genera closely related to Palaeogronotoma are parasitoids of the leaf-miner flies (Agromyzidae). In the Miocene site of Rubielos de Mora (Teruel Province, Spain), type locality of the fossil genus Palaeogronotoma, no agromyzid adult specimens or leaves with the distinctive leaf-mines made by their larvae have been found to date. Possibly agromyzid adult specimens are present in collections from this site, as most of the insect groups wait to be monographed (Peñalver et al., 2013). In contrast, three fossil species of leaf-miner flies were described during the first half of the 20th century from Florissant (Evenhuis, 1994; Meyer, 2003): Agromyza praecursor, Melanagromyza prisca and M. tephrias. However, these species need to be restudied.
Family Cynipidae Billberg, 1820
Subfamily Cynipinae Billberg, 1820
Tribe Aulacideini Nieves-Aldrey, Nylander & Ronquist, 2015
Genus Aulacidea Ashmead, 1897
Aulacidea rotundata (Statz) n. comb.
Figites rotundatus Statz, 1938
Hodiernocynips rotundatus (Statz) Kovalev, 1994
(Figs. 2 & 3)
|
 Fig. 2.—Camera lucida drawings of the female cynipid holotype (nº 3954) of Aulacidea rotundata (Statz, 1938) n. comb., part, from Rott-am-Siebengebirge (Germany). Fig. 2.—Camera lucida drawings of the female cynipid holotype (nº 3954) of Aulacidea rotundata (Statz, 1938) n. comb., part, from Rott-am-Siebengebirge (Germany).
|
|
|
 Fig. 3.—Photographs of the female cynipid holotype (nº 3954) of Aulacidea rotundata (Statz, 1938) n. comb., from Rott-am-Siebengebirge (Germany). 1) Habitus as preserved (the part under alcohol). 2) Counterpart under dry conditions. 3) Head and mesosoma of the counterpart under alcohol showing the notaulices (black arrows) and the pronotal plate (white arrow). 4) Detail of the pronotal plate (white arrow) in the part under alcohol. 5) Metasoma showing a well-preserved, curved ovipositor (the part under alcohol). 6) Forewing venation preserved in the part and photographed under alcohol. Fig. 3.—Photographs of the female cynipid holotype (nº 3954) of Aulacidea rotundata (Statz, 1938) n. comb., from Rott-am-Siebengebirge (Germany). 1) Habitus as preserved (the part under alcohol). 2) Counterpart under dry conditions. 3) Head and mesosoma of the counterpart under alcohol showing the notaulices (black arrows) and the pronotal plate (white arrow). 4) Detail of the pronotal plate (white arrow) in the part under alcohol. 5) Metasoma showing a well-preserved, curved ovipositor (the part under alcohol). 6) Forewing venation preserved in the part and photographed under alcohol.
|
|
Occurrence: Rott-am-Siebengebirge fossil beds (Germany). Latest Oligocene (Chattian) or earliest Miocene (Aquitanian) (for more details see Wappler, 2010).
Holotype: Part and counterpart (Figs. 3.1 & 3.2) of an alate female adult, nº 3954, in two small slabs of oil shale adhered to a wood plate with dimensions 6 × 3.5 × 0.5 cm, housed at the Natural History Museum of Los Angeles (California, USA). Specimen in lateral position, partially incomplete and badly preserved (the two carbonaceous films which constitute it are strongly cleared), having lost some portions of the wings.
Redescription: Female. Body length ca. 3.46 mm. Antennae 1.91 mm long, 13-segmented (according to the original description; currently this character is obscured). Mesosoma structure badly preserved (Fig. 3.3) because the surface is cracked, without microsculpture detected in the cuticle surface. Pronotum long, with admedial depressions narrow forming a continuous groove (Fig. 3.3). Mesosoma
1.38 mm long and 1.30 mm high. Pronotal plate present (Figs. 3.3 & 3.4). Notaulices percurrent (Figs. 2 & 3.3), scutellar foveae not preserved (Fig. 3.3). Forewing long, 3.20 mm estimated length, 1.00 mm greatest width, rounded apically. Wing venation preserved (Figs. 2 & 3.6). Radial cell opened; about three times longer than wide (estimated by continuity of the partially preserved veins to the costal wing margin); Rs+M vein located above the lower part of the basal vein; areolet large. Hindwing 0.32 mm greatest width, with rounded apex. Metasoma globulous, 1.89 mm long and 1.08 mm high, nucha short. Ovipositor well--preserved (Figs. 2 & 3.5), curved, 1.92 mm long.
Comments: The position of Rs+M vein prevents this taxon from being considered a representative of the family Figitidae as it was in the original description; actually belongs to Cynipidae. Kovalev (1994, 1995) included this species, without examining the type material, in the fossil cynipid genus Hodiernocynips, which he described, in 1994, from the upper Oligocene of Maritime Territory (Russian Federation). Nevertheless, the species from Rott-am-Siebengebirge does not belong to Hodiernocynips because the radial cell is opened (closed in that genus) and the prononotum is wide (anterior margin almost equal to height of lateral sides). The pronotal plate is developed, thus the specimen is not a Cynipini, but an Aulacideini as Ronquist et al. (2015) already stated. In the original description by Statz (1938), the radial cell was defined as closed and the areolet was considered small, but after examining the type material the conclusion is that these characters were erroneously interpreted. We observed that the radial cell is opened and the areolet is long (Figs. 2 & 3.6). The relevant characters shown by the specimen fit well with those present in the extant genus Aulacidea, as occurs for the other two species described from Rott; see the “Discussion” section for details. The number of antennal segments, according to our morphological study of the other species described by Statz (1938), maybe was originally misinterpreted and most likely this species had 14-segmented antennae as occurs in A. plana n. comb. (see below).
Aulacidea plana (Statz) n. comb.
Figites planus Statz, 1938
Hodiernocynips planus (Statz) Kovalev, 1994
(Figs. 4 & 5)
|
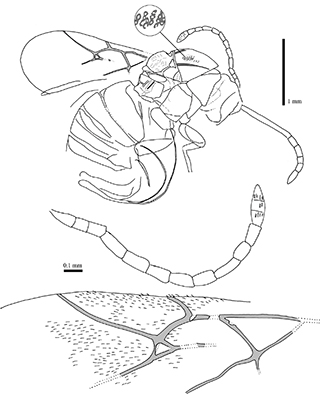 Fig. 4.—Camera lucida drawings of female holotype specimen Aulacidea plana (Statz, 1938) n. comb., nº 3953, part, from Rott-am-Siebengebirge (Germany). Detail from the mesosoma showing the reticulation as linear microsculpture constituted by small pits. Fig. 4.—Camera lucida drawings of female holotype specimen Aulacidea plana (Statz, 1938) n. comb., nº 3953, part, from Rott-am-Siebengebirge (Germany). Detail from the mesosoma showing the reticulation as linear microsculpture constituted by small pits.
|
|
|
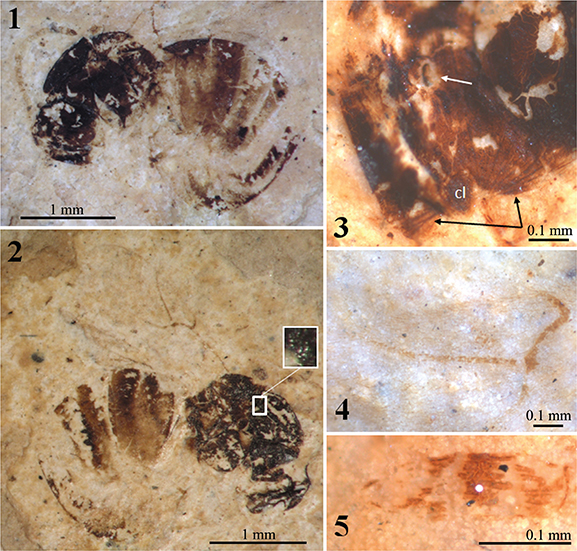 Fig. 5.—Photographs under alcohol of the female cynipid holotype (nº 3953) of Aulacidea plana (Statz, 1938) n. comb., from Rott-am-Siebengebirge (Germany). 1–2) Habitus as preserved in the counterpart (1) and part (2) (inset shows the linear microsculpture constituted by small pits). 3) Detail of the head linear microsculpture (black arrows) and a deformed antennal foramen (white arrow). 4) Forewing radial cell. 5) Antennal club showing three groups of placodeal sensilla. Abbreviation: cl = clypeus. Fig. 5.—Photographs under alcohol of the female cynipid holotype (nº 3953) of Aulacidea plana (Statz, 1938) n. comb., from Rott-am-Siebengebirge (Germany). 1–2) Habitus as preserved in the counterpart (1) and part (2) (inset shows the linear microsculpture constituted by small pits). 3) Detail of the head linear microsculpture (black arrows) and a deformed antennal foramen (white arrow). 4) Forewing radial cell. 5) Antennal club showing three groups of placodeal sensilla. Abbreviation: cl = clypeus.
|
|
Occurrence: Rott-am-Siebengebirge fossil beds (Germany). Latest Oligocene (Chattian) or earliest Miocene (Aquitanian) (for more details see Wappler, 2010).
Holotype: Part and counterpart (Figs. 5.1 & 5.2) of an alate female adult, nº 3953, in two small slabs of oil shale adhered to a wood plate with dimensions 6 × 3.5 × 0.5 cm, housed at the Natural History Museum of Los Angeles (California, USA). Specimen in lateral position, partially incomplete and badly preserved (the two carbonaceous films which constitute it are strongly cleared).
Redescription: Female. Body length 3.44 mm. Antennal foramen visible (Fig. 5.3). Head with linear microsculpture, irradiant carinae from clypeus visible (Fig. 5.3). Antennae 1.59 mm long, with 14 segments (13 segments erroneously indicated in the original description). Flagellomeres longer than broad; F1 slightly longer than pedicel and shorter F2; F3–F7 two or more times as long as wide;
3-segmented apex poorly differentiated, but segments distinguished by presence of placodeal sensilla (Fig. 5.5). Mesosoma structure well-preserved. Mesosoma 1.28 mm long and 1.09 mm high. Pronotal plate present but poorly preserved, obscured. Mesosoma reticulated linear microsculpture constituted by small pits- (Figs. 4 & 5.2); linear elements not visible. Notaulices percurrent (Fig. 4). Mesopleura visible, but its surface shows fine cracks due to deterioration, thus microsculpture is not discernible if present. Scutellar foveae short. Forewing 2.30 mm estimated length, 1.00 mm greatest width, rounded apically. Forewing venation and microtrichia (placodal sensilla) well-preserved (Fig. 4). Radial cell opened (Figs. 4 & 5.4); about three times longer than wide (internal measures); areolet present; Rs+M vein located above the lower part of the basal vein; areolet moderate in size. Hindwing not preserved. Metasoma globulous, ca. 1.65 mm long and ca. 1.20 mm high. Ovipositor well-preserved, curved, 2.30 mm long.
Comments: As in the case of Aulacidea plana n. comb., the position of Rs+M in this specimen prevents it from being considered a figitid; actually, also belongs to the Cynipidae. Kovalev (1994, 1995) also included this species, without examining the type material, in the fossil cynipid genus Hodiernocynips. The opened radial cell indicates that the species from Rott-am-Siebengebirge belongs to a different genus. The pronotal plate is present (but poorly preserved); it is relatively long dorsally, therefore this taxon was transferred to Aulacideini accoding to Ronquist et al. (2015). This species is similar to Aulacidea rotundata n. comb., nevertheless, the number of flagellomeres, the size of the areolet and the size of the radial cell of forewing are different. The number of antennal segments in A. rotundata n. comb. is 13 according to the original description; currently, this feature is not visible as revealed our examination of the fossil specimen. In contrast, A. plana n. comb. clearly has 14 segments (not 13 as indicated in the original description). Also, in the original description, Statz (1938) indicated that the radial cell is closed. However, we observed that actually it is opened. In the “Discussion” section, the placement of this taxon in the genus Aulacidea is explained.
Aulacidea spiniger (Statz) n. comb.
Figites spiniger Statz, 1938
Hodiernocynips spiniger (Statz) Kovalev, 1994
(Figs. 6 & 7)
|
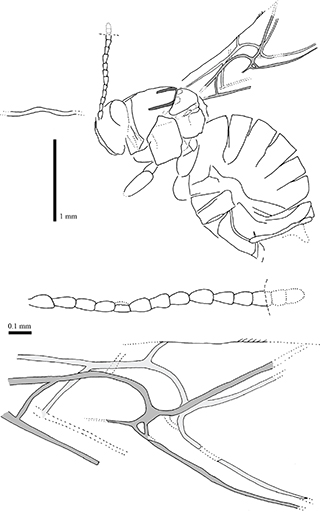 Fig. 6.—Camera lucida drawings of female holotype specimen Aulacidea spiniger (Statz, 1938) n. comb., nº 3955, part, from Rott-am-Siebengebirge (Germany). Fig. 6.—Camera lucida drawings of female holotype specimen Aulacidea spiniger (Statz, 1938) n. comb., nº 3955, part, from Rott-am-Siebengebirge (Germany).
|
|
|
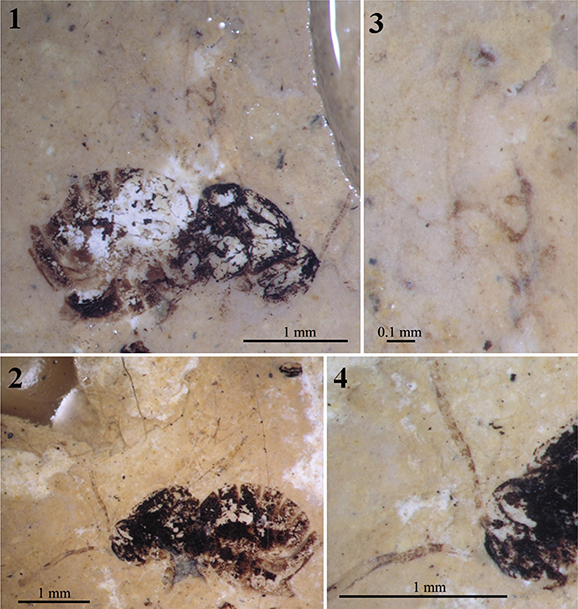 Fig. 7.—Photographs under alcohol of the female holotype specimen Aulacidea spiniger (Statz, 1938) n. comb., nº 3955, from Rott-am-Siebengebirge (Germany). 1–2) Habitus preserved in the counterpart (1) and part (2). 3) Wing venation as preserved in the counterpart. 4) Head and proximal segments of the antennae as preserved in the part. Fig. 7.—Photographs under alcohol of the female holotype specimen Aulacidea spiniger (Statz, 1938) n. comb., nº 3955, from Rott-am-Siebengebirge (Germany). 1–2) Habitus preserved in the counterpart (1) and part (2). 3) Wing venation as preserved in the counterpart. 4) Head and proximal segments of the antennae as preserved in the part.
|
|
Occurrence: Rott-am-Siebengebirge fossil beds (Germany). Latest Oligocene (Chattian) or earliest Miocene (Aquitanian) (for more details see Wappler, 2010).
Holotype: Part and counterpart (Figs. 7.1 & 7.2) of an alate adult, nº 3955, apparently a female, in two small slabs of oil shale adhered to a wood plate with dimensions 6 × 3.5 × 0.5 cm, housed at the Natural History Museum of Los Angeles (California, USA). Specimen in lateral position, partially incomplete and badly preserved (the two carbonaceous films which constitute it are strongly cleared), having lost some portions of the wings and antennae.
Redescription: Female. Body length 3.06 mm. Antennae incomplete (1.18 mm estimated length), with 14 segments (estimated, see the comments below). Flagellomeres longer than broad; F1 longer than wide; F3–F7 shorter than two times as long as wide. Apical flagellomeres lost. Mesosoma structure well-preserved, reticulated. Mesosoma 1.06 mm long and 1.00 mm high. Pronotal plate present but poorly preserved, obscured. Notaulices probably percurrent, not preserved in the anterior part (Figs. 6 & 7.2). Mesopleura cracked due to deterioration. Forewing 2.40 mm estimated length, incomplete. The two forewing venations partially overlapped and well-preserved (Figs. 6 & 7.3). Radial cell opened (Fig. 7.3); about three times longer than wide (internal measures); areolet small; Rs+M vein located above the lower part of the basal vein. Hindwings not preserved. Metasoma globulous (Figs. 6 & 7.1), 1.70 mm long and 1.46 mm high. Ovipositor obscured.
Comments: The erroneous original assignment to the family Figitidae and subsequent assignment to the genus Hodiernocynips is the same than in the two previous taxa discussed. It was also transferred to Aulacideini after Ronquist et al. (2015) as the the pronotum is relatively long dorsally. Aulacidea spiniger n. comb. is very similar to Aulacidea plana n. comb.; despite the wing venations and general body structures that are strongly similar, antennae differ in their thickness and length, and in the shape of their flagellomeres. Originally, this species was described as having 13 antennal segments, but we have commented above that Statz (1938) mistook the number of antennal segments in another specimen. We cannot rule out that the same mistake occurred with this species, however it is possible that A. spiniger n. comb. could have also 14-segmented antennae. These differences, after a careful examination of the type specimens, cannot be attributed to fossil diagenetic deformation. Due to this reason we suspect that, although both species lived at the same period and belong to the same deposit, they must be considered as distinct species, in concordance with the original taxonomical decision by Statz. In the next main section, the placement of this taxon in the genus Aulacidea is provided.
Note on palaeobiology: Cynipids are known as gall wasps. They are endophytophagous herbivores whose larvae develop in galls induced on host plants, either as gall-inducers or as inquiline inhabitants of galls induced by others (Liljeblad & Ronquist,1998; Csóka et al., 2005; Liljeblad et al., 2008; Pénzes et al., 2009; Ronquist et al., 2015); a few unusual species appear to be seed feeders as well (Buffington & Morita, 2009). Most of the species of the genus Aulacidea form galls, usually in stems, but also in flower heads and leaves of the family Asteraceae (Acroptilon, Arnica, Cousinia, Echinops, Eryngium, Hieracium, Koelpinia, Lactuca, Phlomis, Saussurea, Serratula, Sonchus, Silybum, Scorzonera and Tragopogon genera). Aulacidea has a Holarctic distribution; most species are present in Western Palearctic (Melika, 2006).
†Kinseycynipsini n. tribe
Type genus: Kinseycynips Liu and Engel, 2007.
Habitus: Female, Fig. 19 (pg. 30) in Liu et al. (2007).
Diagnosis (female): Clypeus ventrally projecting over mandibles, facial carinae radiating from clypeus present and reaching the compound eye, antennae 14-segmented, F1 slightly curved, all flagellomeres with placodeal sensilla, pronotum broad (ratio of median to posterior distance between dorsal and ventral margins ca. 0.56) without any trace of pronotal plate, mesoscutum and mesopleuron smooth and glabrous, radial cell closed in margin, tarsal claws simple, first metasomal segment crescent-shaped and metasomal T2–T3 free. Male unknown.
Occurrence: Only one fossil species known; it was found in Eocene amber of the Baltic region (ca. 44 MY).
Palaeobiology: The fossil species lived in a palaeoenvironment that has been described by Sadowski et al. (2016), based on vegetal remains preserved in the Baltic amber, as “coastal swamps, back swamps and riparian forests, as well as mixed-mesophytic conifer–angiosperm forests with meadows and open areas” under “a warm-temperate humid climate”.
The fossil genus Kinseycynips could be gallicolous or inquiline; there is no clear evidence, although Liu et al. (2007) affirmed that the species where gallers of herbaceous species of the family Rosaceae. The same authors described the first genera of Eucoilinae (Anteucoila Liu & Engel and Jerseucoila Liu & Engel) as having free metasomal tergae II–IV (fused in all living species), however Ronquist (1999) suggested that K. succinea could be most likely an inquiline species similar to the Synergini (for example), but with free metasomal segments II–III.
Comments: Kinseycynips is a genus erected by Liu et al. (2007) after the re-description of one species from the Eocene amber from the Baltic region. Currently, K. succinea (Kinsey, 1919) has some characters that do not fit within Aulacidea like the 14-segmented antennae (13-segmented in Aulacidea), vertex and mesoscutum glabrous (both pubescent in Aulacidea), and smooth mesopleuron (sculptured in Aulacidea). Liu et al. (2007) also indicated that Kinseycynips is closer to the Rosaceae-galling genera (nowadays included in the tribe Diastrophini, after Ronquist et al., 2015). However, it lacks pronotal plate, and the tarsal claws are simple, unlike in Diastrophini, and has more flagellomeres (just
10 in Diastrophini). On the other hand, Ronquist (1999) suggested that the species described by Kinsey, in 1919, belongs to the genus Synergus (Synergini sensu stricto after Ronquist et al., 2015), but its first metasomal segment is crescent-shaped and the metasomal tergae II and III are free, unlike in Synergini, which has the first metasomal segment anelliform and the metasomal tergae II and III fused. The species shows its face with irradiant carinae from clypeus without two carinae more impressed from toruly to clypeus delimiting a depressed area. These characters discard the inquiline genera Synophromorpha and Periclistus, thus discard the tribe Diastrophini, and also the genus Ceroptres of the tribe Ceroptresini after Ronquist et al. (2015). According to these data, Kinseycynips should be related to the tribes Phanacidini, Aylacini and Aulacideini. The relative length of the pronotum and the absence of any trace of pronotal plate indicate that Kinseycynips is close to the tribe Phanacidini. However, the members of this tribe present the scutum and mesopleuron sculptured, circumstance that also separates Kinseycynips from the other two mentioned tribes.
The unique set of characters within Cynipidae that shows this species allows us to create a new tribe. The well preservation in amber of the sole specimen known and the detailed re-description that Liu and Engel did in Liu et al. (2007) guarantees the validity of this new fossil taxon, despite that the holotype has not been reviewed directly by us. This nomenclatural act is also well-supported by the recent, detailed revision of the gall wasps, done by Ronquist et al. (2015), which clarified the diversity of tribes.
DiscussionTOP
A total of 12 fossil species of Figitidae and 11 of Cynipidae are known after this research (Tables 1 & 2). After the restudy of the type specimen of Figites solus it has been placed, without complete confidence, in the fossil genus Palaeogronotoma, recently described from Miocene lacustrine rocks of Spain. On the other hand, the three Rott species, as described by Statz, definitely are not of the genus Figites, although at the time of the original description, Figites was a very broadly defined genus. One interesting, virtually complete female figitid from the early Eocene Okanagan Highlands of western North America has been recently figured, but not described as a new taxon, by Archibald et al. (2018); due to the scarce fossil record of the family Figitidae, the future study of this well-preserved specimen should be considered of great interest.
Table 1.—Updated list of fossil figitid species indicating some key data. Based on the taxonomic list by Liu et al. (2007).
| FOSSIL FIGITIDAE |
| Subfamily |
Species |
Age |
Locality / Country |
Deposit type |
| Rasnicynipinae |
Rasnicynips eximia |
Upper Cretaceous |
Taimyr (Siberia) |
amber |
| |
(Kovalev, 1994) |
(Santonian) |
Russian Federation |
|
| Palaeocynipinae |
Palaeocynips arcticus |
Upper Cretaceous |
Taimyr (Siberia) |
amber |
| |
Kovalev, 1994 |
(Santonian) |
Russian Federation |
|
| |
Palaeocynipiana santonica |
Upper Cretaceous |
Taimyr (Siberia) |
amber |
| |
Kovalev, 1994 |
(Santonian) |
Russian Federation |
|
| Charipinae |
Protocharips evenhuisi |
Upper Cretaceous |
Taimyr (Siberia) |
amber |
| |
Kovalev, 1994 |
(Santonian) |
Russian Federation |
|
| Eucoilinae |
Anteucoila delicia |
Upper Cretaceous |
Grassy Lake (Alberta) |
amber |
| |
Liu & Engel, 2007 |
(Campanian) |
Canada |
|
| |
Jerseucoila plesiosoma |
Upper Cretaceous |
Sayreville (New Jersey) |
amber |
| |
Liu & Engel, 2007 |
(Turonian) |
USA |
|
| |
Syneucoila magnifica |
Upper Cretaceous |
Sayreville (New Jersey) |
amber |
| |
Liu & Engel, 2007 |
(Turonian) |
USA |
|
| |
Palaeogronotoma nordlanderi |
early Miocene |
Rubielos de Mora |
compression |
| |
Peñalver, Fontal-Cazalla & Pujade-Villar, 2013 |
(early Burdigalian) |
Spain |
(shales) |
| |
Palaeogronotoma? sola |
Late Eocene |
Florissant (Missouri) |
compression |
| |
(Brues, 1910) n. comb. |
|
USA |
(shales) |
| Figitinae |
Palaeofigites balticus |
Eocene |
Baltic deposits |
amber |
|
Kovalev, 1995 |
(Lutetian) |
Baltic Region |
|
| Aspicerinae |
Palaeoaspicera orientalia |
Upper Cretaceous |
Taimyr (Siberia) |
amber |
|
Kovalev, 1994 |
(Santonian) |
Russian Federation |
|
| Subfamily indet. |
Micropresbyteria caputipressa |
Upper Cretaceous |
Grassy Lake (Alberta) |
amber |
| |
Liu & Engel, 2007 |
(Campanian) |
Canada |
|
Table 2.—Updated list of fossil cynipid species indicating some key data. Based on the taxonomic list by Liu et al. (2007).
| FOSSIL CYNIPIDAE |
| Subfamily |
Species |
Age |
Locality / Country |
Deposit type |
| Hodiernocynipinae |
Hodiernocynips primigenius |
Late Eocene |
Bol’shaya Svetlovodnaya |
compression |
| |
Kovalev, 1994 |
(Priabonian) |
Russian Federation |
(diatomite) |
| |
Hodiernocynips progenitrix |
Late Eocene |
Florissant (Missouri) |
compression |
| |
(Kinsey, 1919) |
|
USA |
(shales) |
| |
Hodiernocynips ampliforma |
Late Eocene |
Florissant (Missouri) |
compression |
| |
(Kinsey, 1919) |
|
USA |
(shales) |
| Cynipinae (tribe Aulacideini) |
Aulacidea plana |
Latest Oligocene (Chattian)/ |
Rott-am-Siebengebirge |
compression |
| |
(Starz, 1938) n. comb. |
earliest Miocene (Aquitanian) |
Germany |
(shales) |
| |
Aulacidea rotundata |
Latest Oligocene (Chattian)/ |
Rott-am-Siebengebirge |
compression |
| |
(Starz, 1938) n. comb. |
earliest Miocene (Aquitanian) |
Germany |
(shales) |
| |
Aulacidea spiniger |
Latest Oligocene (Chattian)/ |
Rott-am-Siebengebirge |
compression |
| |
(Starz, 1938) n. comb. |
earliest Miocene (Aquitanian) |
Germany |
(shales) |
| Cynipinae (Kinseycynipsini n. tribe) |
Kinseycynips succinea |
Eocene |
Baltic deposits |
amber |
| |
(Kinsey, 1919) |
(Lutetian) |
Baltic Region |
|
| Cynipinae (tribe Diastrophini) |
Periclistus? vectensis |
Late Eocene |
NW Isle of Wight |
compression |
| |
(Cockerell, 1921) n. comb. |
|
UK |
(limestones) |
| Cynipinae (tribe Diplolepidini) |
Diplolepis? vetus |
Late Eocene |
NW Isle of Wight |
compression |
| |
(Cockerell, 1921) |
|
UK |
(limestones) |
| Subfamily indet. |
Tanaoknemus ecarinatus |
Upper Cretaceous |
Medicine Hat (Alberta) |
amber |
| |
Liu & Engel, 2007 |
(Campanian) |
Canada |
|
| |
“Cynips” succinea |
Eocene |
Baltic deposits |
amber |
| |
Presl, 1822 |
(Lutetian) |
Baltic Region |
|
The three Rott fossil species (all represented by females) belong to Cynipidae, and according to their morphological features (first metasomal tergum is crescent-shaped, dorsomedial pronotum width is long and metasomal tergae are free) they were herb-galling, but not belonging to the fossil subfamily Hodiernocynipinae (according to the previous comments about these species). Ronquist et al. (2015) proposed different tribes for the large group of herb-galling genera previously included in a paraphyletic group named ‘Aylacini’;
these tribes are Diastrophini, Phanacidini, Aylacini and Aulacideini. The tribe Diastrophini include females characterized to have 10 antennal flagellomeres and metasomal tergae II and III fused (but 11–12 flagellomeres and free metasomal tergae in the other tribes, and in the examined fossils as well). The Rott species studied here not belong to the tribe Phanacidini because this taxon does not have pronotal plate (present at least partially in Aylacini, Aulacideini and the examined fossils). Finally, the Rott species belong to Aulacideini as they have long dorsomedial pronotum (1/3–1/4 as long as greatest length of outer lateral margin, and admedian depressions usually round or oval, commonly widely separated, while in Aylacini the pronotal length is shorter -around 1/5-, and admedian depressions are strongly transverse and narrowly separated).
Aulacideini include nine extant genera. The fossils are not Cecconia neither Anistrophus, because the malar space is not at least as long as height of eye in females. Also, they not belong to Liposthenes because the F1 of females is no longer than F2, and they do not belong to Rhodus neither Panteliella because the notauli are not incomplete (distinctly only in the posterior half). Isocolus, Aulacidea and Neaylax are the remaining potential genera. According to Liljeblad & Ronquist (1998), Ronquist & Liljeblad (2001) and Nylander et al. (2004), the genus Isocolus belongs to one of the most primitive phylogenetic lineages of cynipids, the Isocolus-Neaylax lineage. Phylogenetically and morphologically, Isocolus is most closely related to the genera Aulacidea and Neaylax (some diagnostic characters of these genera are presented in Table 3). The genus Neaylax is discarded because its extant species do not have the Rs vein short (not reaching the wing margin) and the maximum number of flagellomeres is 11. On the other hand, there are only few species of Isocolus and Aulacidea which possess a pure combination of diagnostic characters (Melika, 2006); there are some species with opposite characters in Isocolus or in Aulacidea or in both genera (Table 3). According to Melika (2006), most likely only two morphological characters could define the two genera: 1) forewing radial cell is opened (Isocolus) or closed (Aulacidea) (see below the comments about this character in the Rott fossils); and 2) the sculpture of the scutum shows more or less strong transverse striae (Isocolus; in all species) or it is alutaceous or delicately coriaceous (Aulacidea; except in two species, see Table 3).
Table 3.—Key morphological characters of Aulacidea, Neaylax and Isocolus genera compared with Rott fossils. In bold are marked the concordances between extant genera and the fossils studied here.
| Morphological characters |
Aulacidea |
Neaylax |
Isocolus |
fossils |
| R1 reaching wing margin |
30/0 |
1/2 |
2/21 |
+ |
| Rs reaching wing margin |
29/1 |
0/3 |
1/22 |
+ |
| Rs projected in margin |
30/0 |
0/3 |
1/22 |
- |
| Scutum with more or less distinct transverse striae |
2/28 |
0/3 |
22/0 |
- |
| Scutum with coriaceous to alutaceous sculpture |
28/2 |
3/0 |
2/19 |
+ |
| Notauli complete |
25/5 |
1/2 |
21/2 |
+ |
| Number of flagellomeres |
10-12 |
10-11 |
11-12 |
12 |
| F1 shorter than F2 |
5/25 |
3/0 |
20/1 |
+ |
| + = presence, - = absence, x/y = x is the number of species with that character and y is the number of species without that character |
The most important discrepancy in respect to the description of the Rott specimens by Statz is the clearly opened radial cells that show the three specimens. This observation done under high magnification using a stereomicroscope is not attributable to subsequent degradation of the specimens since the original study by Statz. Clearly, the venations of these three specimens became degraded only slightly through decades, as most of them appear intact, and thus the opened radial cell is an original character of the fossils. Furthermore, these specimens are constituted by a part and a counterpart in the Natural History Museum of Los Angeles collection, thus fragments of veins were not lost, as commonly occur when counterparts are discarded in the deposits during collecting. In Rott fossils, the radial cell is opened at the margin, but the R1 and Rs reach the marginal wing, understood to be a trait occurring previous to the closure of the radial cell, as occurs in all Aulacidea species (Table 3); most of the species of Isocolus have these veins far from the wing margin (Table 3). Furthermore, the mesoscutum sculpture in the fossils does not have transverse striae as it occurs in Isocolus and very few species of Aulacidea (Table 3), and F1 is shorter than F2 as generally occurs in the genus Isocolus and in some species of Aulacidea. For all these reasons, we conclude that Rott fossil specimens better fit into Aulacidea genus than into Isocolus. The possibility to describe a new genus for the fossils according to the wing characters is rejected until the correct limits of Aulacidea and Isocolus are established.
The species Aulacidea plana (Statz, 1938) n. comb. is morphologically very similar to Aulacidea spiniger (Statz, 1938) n. comb. They share an identical body size, wing venation, mesosomal structure and metasomal features, nevertheless the comparative lengths of the flagellomeres are very different. Aulacidea rotundata (Statz, 1938) n. comb. is a clearly distinct species morphologically different to the two above mentioned species (see descriptions).
Only two of the 12 fossil species of Figitidae are described from compression deposits to date (Palaeogronotoma nordlanderi and P.? sola n. comb.), both belonging to the subfamily Eucoilinae (Table 1). In contrast, most of the fossil Cynipidae are compression specimens; three of them not classified at tribe level, despite the present review and compilation (Table 2). A hypothetical explanation regarding to Figitidae/amber vs. Cynipidae/compression from the palaeoecological and taphonomic points of view has not been established. As we mention in the “Introduction” section, from figitid/cynipid amber fossils there are available detailed descriptions, unlike compression fossils. Due to this reason the taxonomic placement of compression fossils is sometimes problematic as we have discussed in this study.
Cockerell (1921) described two cynipid species: Rhodites vetus (based on an isolated wing) and Andricus vectensis (based on a specimen lacking antennae) from the Late Eocene limestones of Isle of Wight (UK). Recently, these two species have been cited and figured by Antropov et al. (2014), but not restudied. After Ronquist (1999), Rhodites vetus was assigned to the tribe Diplolepidini; Liu et al. (2007) considered the species as Diplolepis vetus (Cockerell, 1921). However, it is not possible to classify a cynipid isolated wing to the genus level (see Antropov et al., 2014: figure 1.7; note that these authors did not advertise the new status done to this species by Liu et al. in 2007). In consequence, in the present study we consider that the specimen cannot be clearly identified to the genus level, but it can be placed in the subfamily Cynipinae due to the features of its Rs+M vein. We include the species in Table 2 as Diplolepis? vetus.
The species Andricus vectensis has the radial cell closed or partially closed, its metasoma has a big segment occupying most of the metasoma, and its pronotum dorsomedially is long (see Antropov et al., 2014: figure 1.6). According to these features, it cannot be considered as a gall-inducer belonging to the genus Andricus (Cynipini) because in all genera included in the tribe Cynipini the pronotum is short (1/7 or less of outer lateral margin length). According to Ronquist et al. (2015), this species only can be included in Synergini (Synergus or Saphonecrus) or Diastrophini (Periclistus) tribes. Most likely, this species belongs to the extant genus Periclistus because its pronotum is very long medially in respect to Synergus and Saphonecrus, its pronotal plate is visible and its first metasomal tergum is crescent-shaped (vs. Synergus and Saphonecrus, which lack pronotal plate and show first metasomal tergum ring-shaped and longitudinally sulcate). Unfortunately, the specimen lacks antennae (see Crockerell, 1921), being the antennal morphology a key character to establish the genus. We include the species in Table 2 as Periclistus? vectensis n. comb. Furthermore, females of Periclistus have the third valvula of ovipositor outstanding (usually erected) and the scutellum laterally is slightly overhanging the metanotum (also as in Ceroptresini); nevertheless, it does not belong to Ceroptresini because in this tribe the third abdominal tergum is small and free (fused in the fossil specimen).
Tanaoknemus ecarinatus described from Canadian amber (Upper Cretaceous), is a peculiar species according to the characters listed by Liu et al. (2007); this species belonging to an extinct genus has radial cell closed and tarsal claws simple. Within the extant tribes, Aulacideini, Aylacini, Phanacidini, Diplolepidini, Pediaspidini and some Synergini, these two characters occur together. Nevertheless, T. ecarinatus cannot be considered a representative of the tribe Synergini because Synergus/Saphonecrus (the only genera with some species having tarsal claws simple) has the metasomal tergae II and III fused forming a large segment occupying most of the metasoma. In contrast, in T. ecarinatus the metasomal segments are visible. Also, T. ecarinatus is not a representative of Pediaspidini due to its scutellar morphology, and is not a Diplolepidini because its pronotum is wide medially and exhibits different mesopleuron sculpture. Most likely, Tanaoknemus is a basal genus of the old tribe Aylacini (currently cleaved in different new tribes after Ronquist et al., 2015). Nevertheless, Tanaoknemus has peculiar characters not present in these tribes as are: 1) presence of a unique mesopleural impression, 2) unusually long tibiae, 3) very long projection of R1 along the forewing margin after the radial cell, and 4) absence of bulla in Sc+R1 (according to Liu et al., 2007: figure 18). Nevertheless, a revision of the holotype is necessary since several important taxonomical characters to consider in this group after Ronquist et al. (2015) were not originally described (dorsal length of pronotum, pronotal plate, admedian depressions, mesopleural sculpture, etc.); also some others characters (such as the presence of bulla in Sc+R1) show discrepancies between the description and drawings provided and also there are discrepancies between the generic and specific descriptions of characters (e.g., presence or absence of lateral pronotal carina). According to the current data, we refrain from assigning this genus to a particular cynipid subfamily, thus we consider T. ecarinatus as a Cynipidae subfamily indet., as in the original description by Liu et al. (2007).
General conclusionTOP
During the first half of the 20th century, important palaeoentomofaunas were described by renowned scientists as Carpenter, Cockerell, Statz, Brues, Tillyard, Martinov, etc. (Grimaldi & Engel, 2005). However, significant changes occurred later in insect taxonomy and, currently, new techniques and improved microscopes allow a better visualization of tiny characters that were obscure at that time. Together the description of new palaeoentomofaunas which are being discovered abundantly in recent decades, the revision of the ancient collections is mandatory. Both amber and compression fossil insects become degraded in the museums through the decades and a lot of them came from deposits currently inaccessible or less accessible. In consequence, such revision should not be delayed much in time, also if we consider its notable implications for better understanding of the evolution of the most diverse group of organisms.
ACKNOWLEDGEMENTSTOP
We dedicate this work to Dr. Michael Engel (University of Kansas) in recognition of his outstanding contributions to the geological history and evolution of insects, mainly hymenopterans. Thanks are due to Dr. David Grimaldi (American Museum of Natural History) for helped us checking some tiny characters of the Florissant specimen, to Mrs. Bushra Hussaini, of the same institution, for the detailed photographs sent, and to Dr. David Peris (Barcelona University) for his help during the examination of that specimen. Thanks to Dr. Mary Stecheson (Natural History Museum of Los Angeles) for access to Rott specimens and Dr. Torsten Wappler (University of Bonn) to send us important data about the age of the German deposit. Thanks to the two referees, Dr. Alexandr Rasnitsyn (Paleontological Institute, Russian Academy of Sciences) and Dr. Fredrik Ronquist (Swedish Museum of Natural History), for their useful reviews, and to Mr. Marcos Roca Cusachs for correcting the manuscript in English and useful comments.
ReferencesTOP
| ○ |
Antropov, A.V.; Belokobylskij, S.A.; Compton, S.G.; Dlussky, G.M.; Khalaim, A.I.; Kolyada, V.A.; Kozlov, M.A.; Perfilieva, K.S. & Rasnitsyn, A.P. (2014). The wasps, bees and ants (Insecta: Vespida = Hymenoptera) from the Insect Limestone (Late Eocene) of the Isle of Wight, UK. Earth and Environmental Science Transactions of the Royal Society of Edinburgh, 104 (3–4): 335–446. https://doi.org/10.1017/s1755691014000103 |
| ○ |
Archibald, S.B.; Rasnitsyn, A.P.; Brothers, D.J. & Mathewes, R.W. (2018). Modernisation of the Hymenoptera: ants, bees, wasps, and sawflies of the early Eocene Okanagan Highlands of western North America. The Canadian Entomologist: 53 pp https://doi.org/10.4039/tce.2017.59 |
| ○ |
Brues, Ch.T. (1910). The parasitic Hymenoptera of the Tertiary of Florissant, Colorado. Bulletin of the Museum of Comparative Zoology, 54 (1): 3–125. |
| ○ |
Buffington, M.L. & Liljeblad, J. (2008). The Description of Euceroptrinae, a New Subfamily of Figitidae (Hymenoptera), Including a Revision of Euceroptres Ashmead, 1896 and the Description of a New Species. Journal of Hymenoptera Research, 17 (1): 44–56. |
| ○ |
Buffington, M. & Morita, S.I. (2009). Not all oak gall wasps gall oaks: the description of Dryocosmus rileypokei, a new, apostate species of Cynipini from California. Proceedings of the Entomological Society of Washington, 111 (1): 244–253. https://doi.org/10.4289/0013-8797-111.1.244 |
| ○ |
Cockerell, T.D.A. (1921). Fossil arthropods in the British Museum, V. Oligocene Hymenoptera from the Isle of Wight. Annals and Magazine of Natural History, series 9, 7: 1–25. https://doi.org/10.1080/00222932108632485 |
| ○ |
Csóka, G.; Stone, G.N. & Melika, G. (2005). Biology, Ecology and Evolution of gall-inducing Cynipidae. In: Biology, ecology and evolution of gall-inducing arthropods (Raman, A.; Schaefer, C.W. & Withers, T.M., Eds.), Science Publishers, Inc. Enfield, New Hampshire, 569–636. |
| ○ |
Evanoff, E.; Mcintosh, W.C. & Murphey, P.C. (2001). Stratigraphic summary and 40Ar/39Ar geochronology of the Florissant Formation, Colorado. Proceedings of the Denver Museum of Natural Sciences, Ser. 4 (1): 1–16. |
| ○ |
Evenhuis, N.L. (1994). Catalogue of the fossil flies of the world (Insecta: Diptera). Backhuys Publishers, Leiden, 600 pp. |
| ○ |
Fergusson, N.D.M. (1986). Charipidae, Ibaliidae & Figitidae (Hymenoptera: Cynipoidea). Handbooks for the identification of British Insects 8, Part 1c, Royal Entomological Society of London, 55 pp. |
| ○ |
Fergusson, N.D.M. (1995). Cynipoid families. In: The Hymenoptera of Costa Rica (Hanson, P.E. & Gauld, I.D., Eds.), Oxford University Press, Oxford, 243–265. |
| ○ |
Forshage, M. & Nordlander, G. (2008). Identification key to European genera of Eucoilinae (Hymenoptera, Cynipoidea, Figitidae). Insect Systematics & Evolution, 39: 341–359. https://doi.org/10.1163/187631208794760885 |
| ○ |
Gauld, I.D. & Bolton, B. (1988). The Hymenoptera. British Museum, London, 332 pp. |
| ○ |
Goulet, H. & Huber, J.T. (1993). The Hymenoptera of the world: An identification guide to families. Research Branch, Agriculture Canada, Ottawa, 668 pp. |
| ○ |
Grimaldi, D.A. & Engel, M.S. (2005). Evolution of the Insects. Cambridge University Press, New York, 755 pp. |
| ○ |
Harris, R. (1979). A glossary of surface sculpturing. State of California, Department of Food and Agriculture, Occasional Papers in Entomology, 28: 1–31. |
| ○ |
Herman, A.B. (2011). Arman’ Flora of the Magadan Region and Development of Floras in the North Pacific during the Albian–Paleocene. Stratigraphy and Geological Correlation, 19 (1): 71–86. https://doi.org/10.1134/s0869593811010023 |
| ○ |
Kovalev, O.V. (1994). Palaeontological history, phylogeny, and systematics of brachycleistogastromorpha, infraorder N., and cynipomorpha infraorder N. (Hymenoptera) with descriptions of new fossil and recent families, subfamilies, and genera. Entomological Review, 73 (2): 385–426. |
| ○ |
Kovalev, O.V. (1995). Palaeontological history, phylogeny, and systematics of brachycleistogastromorpha, infraorder N., and cynipomorpha infraorder N. (Hymenoptera) with descriptions of new fossil and recent families, subfamilies, and genera. Entomological Review, 74 (4): 105–147. |
| ○ |
Liljeblad, J. & Ronquist, F. (1998). A phylogenetic analysis of higher-level gall wasp relationships (Hymenoptera: Cynipidae). Systematic Entomology, 23: 229–252. https://doi.org/10.1046/j.1365-3113.1998.00053.x |
| ○ |
Liljeblad, J.; Ronquist, F.; Nieves–Aldrey, J.L.; Fontal–Cazalla, F.; Ros–Farré, P.; Gaitros, D. & Pujade–Villar, J. (2008). A fully web-illustrated morphological phylogenetic study of relationships among oak gall wasps and their closest relatives (Hymenoptera: Cynipidae). Zootaxa, 1796: 1–73. |
| ○ |
Liu, Z.; Engel, M.S. & Grimaldi, D.A. (2007). Phylogeny and geological history of the cynipoid wasps (Hymenoptera: Cynipoidea). American Museum Novitates, 3583: 1–48. |
| ○ |
Melika, G. (2006). Gall Wasps of Ukraine. Cynipidae. Vestnik zoologii, supplement 21 (1): 1–300. |
| ○ |
Mertz, D.F.; Renne, P.R.; Wuttke, M. & Mödden, C. (2007). A numerically calibrated reference level (MP28) for the terrestrial mammal-based biozonation of the European Upper Oligocene. International Journal of Earth Sciences, 96 (2): 353–361. https://doi.org/10.1007/s00531-006-0094-6 |
| ○ |
Meyer, H.W. (2003). The Fossils of Florissant. Smithsonian Books, Washington, 258 pp. |
| ○ |
Nieves-Aldrey, J.L. (2001). Hymenoptera Cynipidae. Fauna Ibérica, vol. 16. Ramos, M.A. et al. (Eds.), Museo Nacional de Ciencias Naturales, CSIC, Madrid, 636 pp. http://www.entomologica.es/cont/publis/boletines/1011.pdf |
| ○ |
Nylander, J.A.A. (2004). Bayesian phylogenetics and the evolution of gall wasps. Ph.D. thesis, Acta Universitatis Upsaliensis. Uppsala, 33 pp. |
| ○ |
Pénzes, Z.; Melika, G.; Bozsoki, Z.; Bihari, P.; Mikó, I.; Tavakoli, M.; Pujade–Villar, J.; Fehér, B.; Fülöp, D.; Szabó, K.;
Sipos, B.; Somogyi, K. & Stone, G.N. (2009). Systematic re-appraisal of the gall-usurping wasp genus Synophrus Hartig, 1873 (Hymenoptera: Cynipidae: Synergini). Systematic Entomology, 34: 1–25. https://doi.org/10.1111/j.1365-3113.2009.00482.x |
| ○ |
Peñalver, E. (2002). Los insectos dípteros del Mioceno del Este de la Península Ibérica; Rubielos de Mora, Ribesalbes y Bicorp. Tafonomía y sistemática. Ph.D. thesis, Universitat de València, Valencia, 548 pp. |
| ○ |
Peñalver, E.; Barrón, E.; Postigo-Mijarra, J.M.; García Vives, J.A. & Saura Vilar, M. (2016). El paleolago de Ribesalbes. Un ecosistema de hace 19 millones de años. Diputación de Castellón e IGME, 201 pp. |
| ○ |
Peñalver, E.; Fontal-Cazalla, F.M. & Pujade-Villar, J. (2013). Palaeogronotoma nov. gen. from the Miocene of Spain, the first Tertiary fossil record of the subfamily Eucoilinae (Hymenoptera: Figitidae). Geodiversitas, 35 (3): 643–653. https://doi.org/10.5252/g2013n3a7 |
| ○ |
Pujade-Villar, J. (2002). Comentario Bibliográfico. Una presentación excelente para un volumen con demasiados errores. Nieves-Aldrey, J.L. 2001. Hymenoptera Cynipidae. Fauna Ibérica, vol. 16. Ramos M.A. et al. (Eds), Museo Nacional de Ciencias Naturales. CSIC. Madrid. 636 pp. Boletín de la Asociación Española de Entomología, 26 (3–4): 143–159. http://www.entomologica.es/cont/publis/boletines/1011.pdf |
| ○ |
Ronquist, F. (1995). Phylogeny and early evolution of the Cynipoidea (Hymenoptera). Systematic Entomology, 20 (4): 309–335. https://doi.org/10.1111/j.1365-3113.1995.tb00099.x |
| ○ |
Ronquist, F. (1999). Phylogeny, classification and evolution of the Cynipoidea. Zoologica Scripta, 28 (1–2): 139–164. https://doi.org/10.1046/j.1463-6409.1999.00022.x |
| ○ |
Ronquist, F. & Liljeblad, J. (2001). Evolution of the gall wasp-host plant association. Evolution, 55 (12): 2503–2522. |
| ○ |
Ronquist, F. & Nordlander, G. (1989). Skeletal morphology of an archaic cynipoid, Ibalia rufipes (Hymenoptera: Ibaliidae). Entomologica Scandinavica, Supplement 33: 1–60. |
| ○ |
Ronquist, F.; Nieves-Aldrey, J.L.; Buffington, M.L.; Liu, Z.; Liljeblad, J. & Nylander, J.A.A. (2015). Phylogeny, evolution and classification of gall wasps: The plot thickens. PLoS ONE, 10 (5): 40 pp. ttps://doi.org/10.1371/journal.pone.0123301 |
| ○ |
Ros-Farré, P. & Pujade-Villar, J. (2007). Plectocynipinae, a new subfamily of Figitidae and description of a new Neotropical genus of Thrasorinae (Hymenoptera: Cynipoidea). Zootaxa, 1583: 1–13. https://doi.org/10.11646/zootaxa.1583.1.1 |
| ○ |
Sadowski, E.-M.; Seyfullah, l.j.; Schmidt, A.R. & Kunzmann, L. (2016). Towards a new picture of the ‘Baltic amber forest’. Abstracts 7th International Conference on Fossil Insects, Arthropods and Amber, Edited by Penney, D. & Ross, A.J., Siri Scientific Press, Edinburgh: p. 45. |
| ○ |
Statz, G. (1938). Neue Funde parasitischer Hymenopteren aus dem Tertiar von Rott am Siebengebirge. Decheniana, 98 A: 71–144. |
| ○ |
Todt, W. & Lippolt, H.J. (1980). K–Ar age determination on Tertiary volcanic rocks. V. Siebengebirge-Graben. Journal of Geophysics, 48: 18–27. |
| ○ |
Wappler, T. (2010). Insect herbivory close to the Oligocene–Miocene transition - a quantitative analysis. Palaeogeography, Palaeoclimatology, Palaeoecology, 292: 540–555. https://doi.org/10.1016/j.palaeo.2010.04.029 |
Fig. 1.—Holotype specimen (A60) of Palaeogronotoma? sola n. comb., from Florissant, USA. 1) Original drawing made by Brues (1910). 2) Photograph of the holotype under dry conditions. 3) Same photograph pointing the ventral part of metasoma in the correct position. 4) Similar photograph; insets contain photographs under alcohol showing important morphological characters. Photographs 2–4 taken by Mrs. Bushra Hussaini (Courtesy of AMNH). Abbreviations: F = Flagellomere, MT1 = first tergum of the metasoma.