The Ediacaran-Cambrian transition: sedimentary facies versus extinction
La transición ediacárico-cámbrica: facies sedimentarias versus extinción
J.G. Gehling1, D.C. García-Bellido1, M.L. Droser2, M.L. Tarhan3, B. Runnegar4
1Earth Sciences Section, South Australian Museum, North Terrace, Adelaide, 5000 South Australia, Australia. Email: jim.gehling@samuseum.sa.gov.au; ORCID ID: https://orcid.org/0000-0001-8459-5440, http://orcid.org/0000-0003-1922-9836
2Department of Earth Sciences, University of California at Riverside, Riverside, California, USA. ORCID ID: http://orcid.org/0000-0001-7112-5669
3Department of Geology and Geophysics, Yale University, New Haven, Connecticut 06511, USA. ORCID ID: https://orcid.org/0000-0002-5878-929X
4Department of Earth, Planetary and Space Sciences, University of California, Los Angeles, CA 90095-1567, USA. ORCID ID: http://orcid.org/0000-0002-7412-7982
| |
ABSTRACT
Recent analysis of the terminal Ediacaran, Rawnsley Quartzite, in the Flinders Ranges of South Australia, demonstrates that key taxa of the Ediacara biota are restricted to certain sedimentary facies and stratigraphic levels. The Rawnsley Quartzite consists of three members separated by disconformities: (i) the basal, shallow marine Chace Sandstone Member is unfossiliferous, but replete with textured organic surfaces; (ii) the overlying Ediacara Sandstone Member fills submarine incisions cut through the underlying Chace Quartzite Member and paralic Bonney Sandstone below the Rawnsley Quartzite; and (iii) the Ediacara Sandstone Member is incised by the less fossiliferous Nilpena Sandstone Member that caps the Rawnsley Quartzite.
Keywords: Facies control; Flinders Ranges; Ediacaran; Australia.
|
| |
RESUMEN
Un estudio reciente de la Cuarcita de Rawnsley, en el Ediacárico terminal Ediacaran de la Cordillera de Flinders, Australia meridional, demuestra cómo algunos taxones clave de la biota de Ediacara están restringidos a ciertas facies sedimentarias y determinados niveles estratigráficos. La Cuarcita de Rawnsleycomprende tres miembros separados por discotinuidades: (i) el Miembro basal de la Arenisca de Chace es somera y azoica, aunque destacan las superficies con texturas orgánicas; (ii) el Miemrbo de la Arenisca de Ediacara rellena un Sistema de incisiones submarinas que recortan el miembro inferior de Chace y la Arenisca parálica de Bonney, infrayacente a la Cuarcita de Rawnsley; y (iii) el Miembro de la Arenisca de Ediacara es asimismo recortada de forma erosiva por el Miembro de la Arenisca de Nilpena, menos fosilífera.
Palabras clave: Control de facies; Cordillera de Flinders; Ediacárico; Australia.
|
IntroductionTOP
Claims about global extinction of the Ediacara biota, and the origins and duration of the “Cambrian explosion” of marine animal life, have dominated the literature for decades. In most cases, emphasis is given to key geological sections with datable ash beds and either diverse assemblages of body fossils or trace fossils. However, apart from Buatois et al. (2013) and Gehling & Droser (2013), little attention has been paid to the role of the succession of sedimentary facies responsible for these samples of the fossil record spanning the Ediacaran-Cambrian boundary.
Decades of analyses of the terminal Ediacaran, Rawnsley Quartzite, in the Flinders Ranges of South Australia (Gehling, 2000), demonstrates that key taxa of the Ediacara biota are restricted to certain sedimentary facies and stratigraphic levels.
The Rawnsley Quartzite consists of three members separated by disconformities. The basal, shallow marine, Chace Sandstone Member is unfossiliferous, but replete with textured organic surfaces. The overlying, richly fossiliferous, Ediacara Sandstone Member fills submarine incisions cut through the regional base of the Rawnsley Quartzite, including the underlying tidal sand-flats of the Chace Quartzite Member and the otherwise basal, paralic Bonney Sandstone (Gehling & Droser, 2012; Counts et al., 2016). In turn, the Ediacara Sandstone Member was secondarily incised by the less fossiliferous, Nilpena Sandstone Member (new name), that caps the Rawnsley Quartzite. In the Nilpena area, the base of the Cambrian is marked by a sharp change to the Early Cambrian U-shaped Diplocraterian burrow sands of the Parachilna Formation.
Results and discussionTOP
The oldest recorded fossils, in the Ediacara SS Member of the Rawnsley Quartzite, are Helminthoidichnites isp. groove and levee traces within thinly laminated silty sandstones that inter-tongue with sandstone breccias within a canyon fill in the Nilpena region (Gehling & Droser, 2012) and also in the central Flinders Ranges, where the Chace SS Member is missing. Higher in the Ediacara SS Mbr., Helminthoidichnites groove traces with scalloped levees (Fig. 1a, b), are found on both bed-tops and bed soles. As demonstrated in the Nilpena SS Member (Gehling & Droser, 2018) bed sole grooves and levees were likely made by tiny bilaterians, burrowing beneath thins sand laminae, separated from sand below by microbial mats. These characteristics are exclusive to bed soles of very thin sandstone layers usually less than
5mm thick, but never thicker than 15mm. Identified on both bed-soles and bed-tops of excavated beds, Helminthoidichnites grooves are bordered by scalloped levees (Fig. 1 a, b, c). They appear to have been produced by small, peristaltic-burrowing animals, exploiting organic, mat-bound sands,
both on clearly identified bed tops (Fig. 1c), but also on bed bases (Fig. 1a, b) where mats were smothered by thin sand cover that facilitated sufficient levels of dissolved oxygen (Fig. 1a, b) for the survival of burrowing organisms. These Helminthoidichnites burrows are common in oscillation rippled sandstone facies of the Ediacara SS Member, where crossing avoidance is common,
except where the burrowing organisms were scavenging previously buried bodies (Gehling & Droser, 2018). Bed-sole casts of 3mm wide, bilobed trackways (Fig. 1c), that occur within red, laminated, fine-grained sandstone facies, low in the Ediacara SS Member, suggest furrowing activity by 3 mm wide bilaterian organisms.
|
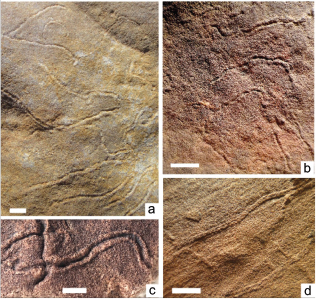 Fig. 1.—a, b: Crossing-avoidance of Helminthoidichnites groove trace fossils on bed soles of thinly bedded, oscillation rippled sandstone of the Ediacara SS Mbr; c: Bed top with Helminthoidichnites crenulated levees; d: Bed-sole casts of bilobate furrows in deeper water, flat laminated, reddish, fine grained sandstone. Scale in centimetres. Fig. 1.—a, b: Crossing-avoidance of Helminthoidichnites groove trace fossils on bed soles of thinly bedded, oscillation rippled sandstone of the Ediacara SS Mbr; c: Bed top with Helminthoidichnites crenulated levees; d: Bed-sole casts of bilobate furrows in deeper water, flat laminated, reddish, fine grained sandstone. Scale in centimetres.
|
|
At both the Nilpena and the Ediacara Hills fossil sites, three-walled, palisade constructed specimens of Pteridinium simplex (Fig. 2a) are preserved in mass-flow sandstones, low in these submarine valley incisions. Pteridinium is absent in overlying shallow marine facies of the Ediacara Sandstone Member, which records the majority of classic members of the Ediacara biota in South Australia. The absence of Pteridinium in the overlying Nilpena SS Member, in South Australia, is in contrast to its stratigraphic range of some 1300m of section in the latest Ediacaran, Schwarzrand Subgroup of southern Namibia (Narbonne et al., 1997). There, Pteridinium ranges from the Kliphook Member (Dabis Formation) to the Spitskop Member (Nomsas Formation) just below the early Cambrian disconformity. This emphasizes the role of environment in determining the record of large, distinctive body fossils let alone casts and moulds of tiny organisms that, until recently, have been overlooked.
|
 Fig. 2.—a: Pteridinium simplex, Ediacara SS Member; b: Phyllozoon hanseni, Nilpena SS Member.; c: Treptichnus pedum, Uratanna Form; d: Unnamed body fossils, Uratanna Form., Castle Rock, Angepina Syncline, Flinders Ranges. Castle Rock, Angepina Syncline, Flinders Ranges. Fig. 2.—a: Pteridinium simplex, Ediacara SS Member; b: Phyllozoon hanseni, Nilpena SS Member.; c: Treptichnus pedum, Uratanna Form; d: Unnamed body fossils, Uratanna Form., Castle Rock, Angepina Syncline, Flinders Ranges. Castle Rock, Angepina Syncline, Flinders Ranges.
|
|
Conversely, gregarious taxa, like Phyllozoon hanseni (Fig. 2b) and Eoandromeda octobrachiata, in addition to Dickinsonia rex, and several, as-yet, undescribed taxa, are exclusive to the fine-grained, sandstone facies of the newly named Nilpena Sandstone Member that fills a second incision and caps the Rawnsley Quartzite in South Australia. However, many characteristic genera,
like Tribrachidium, Parvancorina, Spriggina, Kimberella, Rugoconites and smaller species of Dickinsonia, are common in the oscillation-rippled sandstone facies of both these fossil-bearing members (Droser & Gehling, 2015; Droser et al., 2017; Evans et al., 2018). This demonstrates that classic, large Ediacaran body forms are unlikely to have been missed in early Cambrian sediments of similar environments; rather, with very few exceptions, they did not survive the onset of penetrative burrowing and predation that appears to have characterized the Cambrian explosion in shallow marine, higher energy environments.
Fine-grained and thinly bedded sandstones of both members, at Nilpena and the Ediacara Conservation Park, preserve tiny, relatively featureless, rice-grain-sized body moulds, small enough to have been the animals responsible Helminthoidichnites isp. groove traces (Gehling & Droser, 2018), in addition to very small external moulds of taxa, with affinities to Spriggina, Praecambridium and Kimberella. These levee and groove traces (Fig.1a, b), found on both tops and bases of thinly bedded sandstone laminae, show crossing-avoidance and also preserve evidence of either peristaltic or appendicular displacement of sediment (Figs 1c and 2b). Arguably, several small, and as yet un-named,
body-fossil taxa are candidate Ediacaran “survivors”. External moulds of such organisms are unlikely to be identified from better-oxygenated Cambrian sandy seafloors where active predation and scavenging may have erased their record before the advent of mineral skeletons.
The Rawnsley Quartzite is disconformably overlain by the Uratanna Formation in the northern Flinders Ranges and, in turn,
by the Parachilna Formation. The Uratanna Formation spans the Ediacaran-Cambrian boundary, as defined by the facies-controlled appearance of the characteristic early Cambrian, branching burrows of Treptichnus pedum (Fig. 2c) and at least one bilateral, soft-bodied “holdover” taxon described by Jensen et al. (1998) (Fig. 2d). The Cambrian Diplocraterion burrow-beds of the Parachilna Formation overlie the Uratanna Formation. Where the Uratanna Fm. is not preserved, these Parachilna Fm. burrow beds sharply overly the unfossiliferous Ediacaran sandstone facies of the Rawnsley Quartzite. Since the fossiliferous members of the Rawnsley Quartzite are capped by 50 to 400m of shallow marine, high flow regime sandstone facies, there is no possibility of a record of taxa that may or may not have spanned the Ediacaran-Cambrian boundary.
The records of these key body and trace fossil taxa are a function of appropriate sedimentary facies, rather than an exclusive record of true biostratigraphic ranges of taxa, and thus resulting in placement of the Ediacaran\Cambrian boundary in South Australia within a disconformity. Globally, it is unlikely that any one succession holds a true record of the precise base of the Phanerozoic. Consequently, there may be no single record of extinction of the Ediacara biota, or any record of the specific taxa that ignited the Cambrian explosion of cuticle body-armoured and appendage-bearing marine animals. Large, soft-bodied taxa of the Ediacara biota, such as Dickinsonia, Yorgia, Phyllozoon and Pteridinium, are unlikely to have survived in Cambrian shallow marine settings.
However, recent statistical analysis of the evolution of Cambrian trilobites (Edgecombe et al. 2019, has promoted previously voiced rejection of claims that small-scale, bilaterial Ediacaran taxa, such as Parvancorina, Spriggina, Praecambridium and Kimberella, were likely Ediacaran precursors to the Cambrian explosion of bilaterians, simply because such taxa have not been discovered in dated Early Cambrian strata of appropriate sedimentary composition. The earliest Cambrian fossil Lagerstätten, being limited to deeper water, fine grained sedimentary settings, document the diversification of arthropods and other megascopic animals with mineralized and cuticular skeletons. These appear well above the base of the Cambrian in sections described globally.
The absence of strong evidence of Ediacaran body-fossil holdovers is likely a function of a lack of suitable shallow-marine preservational environments in the Cambrian that were not also subjected to bioturbation. Soft-bodied animals, small shelly fossils, organic-walled microfossils and trace fossils are each confined to markedly different sedimentary facies. This reduces our resolution of the ranges of late-Ediacaran marine animal assemblages and increases the difficulty of assessing taxonomic ranges.
Assemblages recorded by late Ediacaran fossil-bearing successions from several continents, show differing arrays of Ediacara body and trace fossils in these transitions into Early Cambrian strata, depending on the preserved sedimentary settings (see
Narbonne et al., 1997; Jensen & Runnegar, 2005; Darroch et al., 2015; Smith et al., 2017). There are no two, well-separated, successions that show a simple pattern of megascopic species that can be used to infer a specific extinction event at the Ediacaran-Cambrian boundary. The preservation of trace fossils, on one hand, and body fossils of millimetric-scale taxa, such as Praecambridium, large numbers of juvenile specimens of Parvancorina (Coutts et al., 2018), and other as yet undescribed, tiny bilateral forms, is limited by both the grain-size resolution and chemical composition of these Ediacaran and early Cambrian siliciclastic units, prior to the onset of effective bioturbation in the Early Cambrian.
The newly defined Nilpena Sandstone Member, capping the Rawnsley Quartzite in South Australia, is characterized by Phyllozoon hanseni, Eoandromeda octobrachiata, Dickinsonia rex and a new species of Tribrachidium, together with a number of, as yet, undescribed taxa. The new Nilpena Sandstone Member is restricted to the central and southern Flinders Ranges, south of the Ediacara Conservation Park, where the transitional facies of the Uratanna Formation appears to include the Ediacaran-Cambrian transition in the northern Flinders Ranges. The bioturbated sands of the Flinders Ranges wide, Parachilna Formation, cap the succession before the transition to early Cambrian carbonates recording a regional rise in sea level. These facies-fossil relationships in South Australian, act as a reminder that species ranges in most time-equivalent late Ediacaran sedimentary successions, world-wide, reflect similar facies control, and thus cannot be utilized as evidence of global origins and extinctions in any one succession.
In the youngest Ediacaran strata of South Australia, the stratigraphic range of Pteridinium (Fig. 2a), as in Namibia, is determined by the availability of suitable sedimentary facies. Specimens of Pteridinium at Swartpunt (Namibia), just below the Cambrian erosional incision, are flattened. However, three-dimensional Pteridinium specimens (Fig. 2a) within canyon-fill sandstones, near the base of the Ediacara SS Member, cannot be mistaken as taphomorphs of the bedding-surface arrays of two-dimensional Phyllozoon stipes (Fig. 2b), that are exclusive to bedding surfaces in the Nilpena SS Member in South Australia, some 200m stratigraphically above the known horizons of Pteridinium at Nilpena and elsewhere in the Flinders Ranges to the east and south.
The upper part of the Ediacara Sandstone Member, as originally described by Jenkins et al. (1982), at NW Ediacaran Conservation Park, conserves a suite of unpublished small-sized, bilaterian-like taxa, in addition to Praecambridium and tiny specimens of Parvancorina and Dickinsonia and Rugoconites. As yet, such small Ediacaran organisms have not been recognized in the overlying Nilpena Sandstone Member, largely due to subtle differences in the sedimentary settings recorded by each member of the Rawnsley Quartzite. The facies control on preservation of diverse Ediacaran fossil species (see Cohen et al., 2009), and the regional role of disconformities in the upper Rawnsley Quartzite, emphasizes the need for caution in claiming that any one late Ediacaran succession, world-wide, can be used to define the extinction of the Ediacara biota.
The Rawnsley Quartzite is disconformably overlain by incised channel-filling sandstone of the basal Uratanna Formation, sharply overlain by khaki coloured, coarsening, shallowing-upward siltstone and sandstones bearing a rich trace fossil assemblage,
including Treptichnus pedum (Fig. 2c) and T. coronatum, overlain by sandstones including distinctive but un-named, frond-like body fossils (Fig. 2d, Jensen et al., 1998). The Uratanna Formation is capped by a prominent package of shallow-marine, coarse-grained sandstone, overlain by the Parachilna Formation which in turn is dominated by Diplocraterion burrowed sandstone.
ReferencesTOP
| ○ |
Buatois, L.A.; Almond, J. & Germs, G.J.B. (2013). Environmental tolerance and range offset of Treptichnus pedum: Implications for the recognition of the Ediacaran-Cambrian boundary. Geology, 41: 519-522. https://doi.org/10.1130/G33938.1 |
| ○ |
Cohen, P.A.; Bradley, A.; Knoll, A.H.; Grotzinger, J.P.; Jensen, S.; Abelson, J.; Hand, K. Love, G.; Metz, J.; McLoughlin,
N.; Meister, P.; Shepard, P.R.; Tice, M. & Wilson, J.P. (2009). Tubular compression fossils from the Ediacaran Nama Group,
Namibia. Journal of Paleontology, 83: 110-122. https://doi.org/10.1666/09-040R.1 |
| ○ |
Counts, J.W.; Rarity, F.; Ainsworth, R.B.; Amos, K.J.; Lane, T.; Moro’n, S.; Trainor, J.; Valenti, C. & Nanson, R. (2016).
Sedimentological interpretation of an Ediacaran delta: Bonney Sandstone, South Australia. Australian Journal of Earth Sciences,
63: 257-273. https://doi.org/10.1080/08120099.2016.1180322 |
| ○ |
Coutts, F.J.; Bradshaw, C.J.A.; García-Bellido, D.C. & Gehling, J.G. (2018). Evidence of sensory-driven behaviour in the Ediacaran organism Parvancorina: Implications and autecological interpretations. Gondwana Research, 55: 21-29. https://doi.org/10.1016/j.gr.2017.10.009 |
| ○ |
Darroch, S.A.F.; Sperling, E.A.; Boag, T.H.; Racicot, R.A.; Mason, S.J.; Morgan, A.S.; Tweedt, S.; Myrow, P.; Johnston, D.T.;
Erwin, D.H. & Laflamme, M. (2015). Biotic replacement and mass extinction of the Ediacara biota. Proceedings Royal Society B, 282: 20151003. https://doi.org/10.1098/rspb.2015.1003 |
| ○ |
Droser, M.L. & Gehling, J.G. (2015). The advent of animals: the view from the Ediacaran: Proceedings of the National Academy of Sciences, 112: 4865-4870. https://doi.org/10.1073/pnas.1403669112 |
| ○ |
Droser, M.L.; Tarhan, L.G. & Gehling, J.G. (2017). The Rise of Animals in a Changing Environment: Global Ecological Innovation in the Late Ediacaran. Annual Reviews in Earth and Planetary Science, 45: 593-617. https://doi.org/10.1146/annurev-earth-063016-015645 |
| ○ |
Evans, S.D. Dzaugis, P.W.; Droser, M.L. & Gehling, J.G. (2018). You can get anything you want from Alice’s Restaurant Bed:
exceptional preservation and an unusual fossil assemblage from a newly excavated bed (Ediacara Member, Nilpena, South Australia).
Australian Journal of Earth Sciences. https://doi.org/10.1080/08120099.2018.1470110 |
| ○ |
Gehling, J.G. (2000). Sequence stratigraphic context of the Ediacara Member, Rawnsley Quartzite, South Australia: a taphonomic window into the Neoproterozoic biosphere. Precambrian Research, 100: 65-95. https://doi.org/10.1016/S0301-9268(99)00069-8 |
| ○ |
Gehling, J.G. & Droser, M.L. (2012). Ediacaran stratigraphy and the biota of the Adelaide Geosyncline, South Australia. Episodes,
35: 236-246.
|
| ○ |
Gehling, J.G. & Droser, M.L. (2013). How well do fossil assemblages of the Ediacara Biota time? Geology, 41: 447-450. https://doi.org/10.1130/G33881.1 |
| ○ |
Gehling, J.G. & Droser, M.L. (2018). Ediacaran scavenging as a prelude to predation. Emerging Topics in Life Sciences, 2:
213-222. https://doi.org/10.1042/ETLS20170166 |
| ○ |
Jenkins, R.J.F.; Ford, C.H. & Gehling, J.G. (1983). The Ediacara Member of the RawnsleyQuartzite: the context of the Ediacara assemblage (late Precambrian, FlindersRanges). Australian Journal of Earth Sciences, 30: 101-119. https://doi.org/10.1080/00167618308729240 |
| ○ |
Jensen S. & Runnegar, B.N. (2005). A complex trace fossil from the Spitskop Member (terminal Ediacaran-? Lower Cambrian) of southern Namibia. Geological Magazine, 142: 561-569. https://doi.org/10.1017/S0016756805000853 |
| ○ |
Jensen, S.; Gehling, J.G.; & Droser, M.L. (1998). Ediacaran-type fossils in Cambrian sediments. Nature, 393: 567-569. https://doi.org/10.1038/31215 |
| ○ |
Narbonne, G.M.; Saylor, B.Z. & Grotzinger, J.P. (1997). The youngest Ediacaran fossils from southern Africa. Journal of Paleontology,
71: 953-967. https://doi.org/10.1017/S0022336000035940 |
| ○ |
Smith, E.F.; Nelson, L.L.; Tweedt, S.M.; Zeng. H.; and Workman, J.B. (2017). A cosmopolitan late Ediacaran biotic assemblage:
new fossils from Nevada and Namibia support a global biostratigraphic link. Proc. R. Soc. B 284: 20170934. https://doi.org/10.1098/rspb.2017.0934 |
Fig. 1.—a, b: Crossing-avoidance of Helminthoidichnites groove trace fossils on bed soles of thinly bedded, oscillation rippled sandstone of the Ediacara SS Mbr; c: Bed top with Helminthoidichnites crenulated levees; d: Bed-sole casts of bilobate furrows in deeper water, flat laminated, reddish, fine grained sandstone. Scale in centimetres.